Overview
γ-Carboxylation occurs mainly in proteins related to blood coagulation. Glutamic acid residues are carboxylated by the enzyme glutamyl carboxylase in γ-position in the presence of oxygen and carbon dioxide. Vitamin K is required as a cofactor.
| pKa | NC | Loss | Gain | Deltamass | H | AA | UV-Spec | Pattern |
| Acidic 2.03 | Yes | H | CHO2 | Av: 44.0095 M: 43.9898 | – | E | – | – |
In-depth mechanism
γ-Carboxylation is a post-translational modification that is characterstic for a few proteins, mainly proteins that are associated with blood coagulation. In γ-carboxylation, a carboxyl group is added to the γ-carbon atom of a glutamate residue. The addition of the carboxyl group is catalysed by γ-glutamyl carboxylase (GGCX), which is an integral membrane protein. In order to be able to perform the γ-carboxylation, vitamin K as a cofactor is needed. GGCX oxidizes vitamin K hydroquinone in the presence of molecular oxygen to vitamin K epoxide while simultaneously adding carbon dioxide to the γ-carbon atom of a glutamate residue. In the process a water molecule is released. Vitamin K hydroquinone is regenerated in two steps by reduction of vitamin K epoxide via vitamin K epoxide reductase. The result of γ-carboxylation is the non-proteinogenic amino acid carboxyglutamate. Carboxyglutamate is able to selectively and strongly chelate Ca2+-ions because of the two carboxyl groups. This is essential for complete functionality of blood factor VII, IX and X for example1–4. The carboxyl group on the side chain of glutamate resiude has a pKa value of approximately 4.255. For carboxyglutamate, the pKa value of the first carboxylgroup on the side chain remains the same while the second carboxyl group has a lower pKa value of 2.036. Per γ-carboxylation the molecular mass increases by 44 Da7. The mechanism is shown in figure 1.
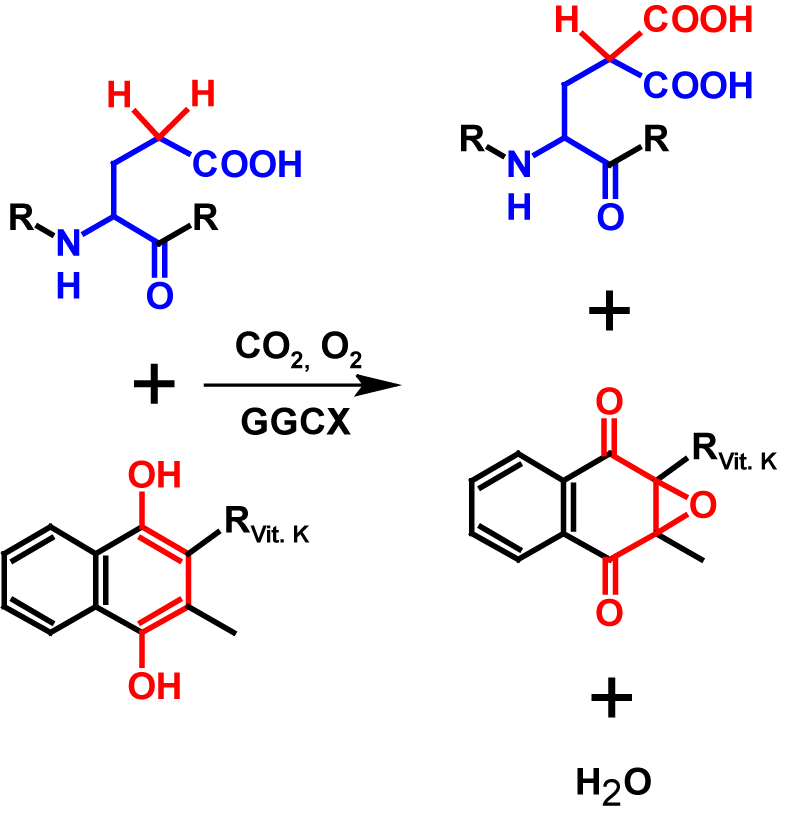
γ-Carboxylation sites are not conserved by a specific consensus sequence, but are rather mediated by directly adjacent propeptide regions, which are subsequently cleaved proteolytically. The propetdide region has a high binding affinity to GGCX and also activates the enzyme. This enables the γ-carboxylation of several glutamate residues during a single binding1–3. Most γ-carboxylated proteins in vertebrates have a so called N-terminal carboxyglutamate domain. This domain is around 45 amino acids long and contains between 9 – 12 glutamate residues which are all γ-carboxylated. These proteins are almost exclusively synthesized in the liver2. Currently, 20 proteins have been discovered that are γ-carboxylated1.
References
- 1.Azuma K, Inoue S. Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders. International journal of molecular sciences. 2019;20. doi:10.3390/ijms20112844
- 2.Hansson K, Stenflo J. Post-translational modifications in proteins involved in blood coagulation. Journal of thrombosis and haemostasis : JTH. 2005;3:2633–2648. doi:10.1111/j.1538-7836.2005.01478.x
- 3.Walsh G, Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nature Biotechnology. 2006;24:1241–1252. doi:10.1038/nbt1252
- 4.Silva PJ, Ramos MJ. Reaction Mechanism of the Vitamin K-Dependent Glutamate Carboxylase: A Computational Study. The Journal of Physical Chemistry B. 2007;111:12883–12887. doi:10.1021/jp0738208
- 5.Craig NL. Molecular Biology: Principles of Genome Function. Second edi. Oxford University Press; 2014.
- 6.Burnier J, Borowski M, Furie B, Furie B. Gamma-carboxyglutamic acid. In: The Biological Effects of Glutamic Acid and Its Derivatives. Martinus Nijhoff/Dr. W. Junk Publishers; 1981:191-207.
- 7.Ramström M, Sandberg H. Characterization of γ-carboxylated tryptic peptides by collision-induced dissociation and electron transfer dissociation mass spectrometry. European journal of mass spectrometry (Chichester, England). 2011;17:497–506. doi:10.1255/ejms.1149