1 Background
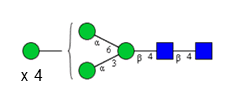
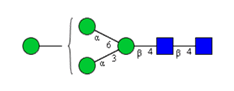
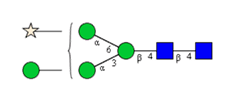
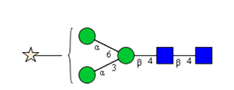
N-glycosylations affect important properties such as the specificity, efficacy, immunogenicity and stability of proteins. A portfolio of methods for glycan analysis was developed, using the murine monoclonal antibody 1RK1, an IgG1κ, to enable N-glycosylations of proteins to be analysed (Fig. 1). The methods developed were subsequently applied to the plant glycoprotein ricin. Ricin contains high-mannose type glycans with additional monosaccharides, such as xylose, and different linkages of monosaccharide building blocks compared to animal glycoproteins. Ricin is a potent toxin from the plant Ricinus communis, which is included on list 1 of the Chemical Weapons Convention because of its high toxicity and easy availability. The toxin was inactivated in the Spiez Laboratory and subsequently analysed at the ZHAW following a pre-developed security concept.
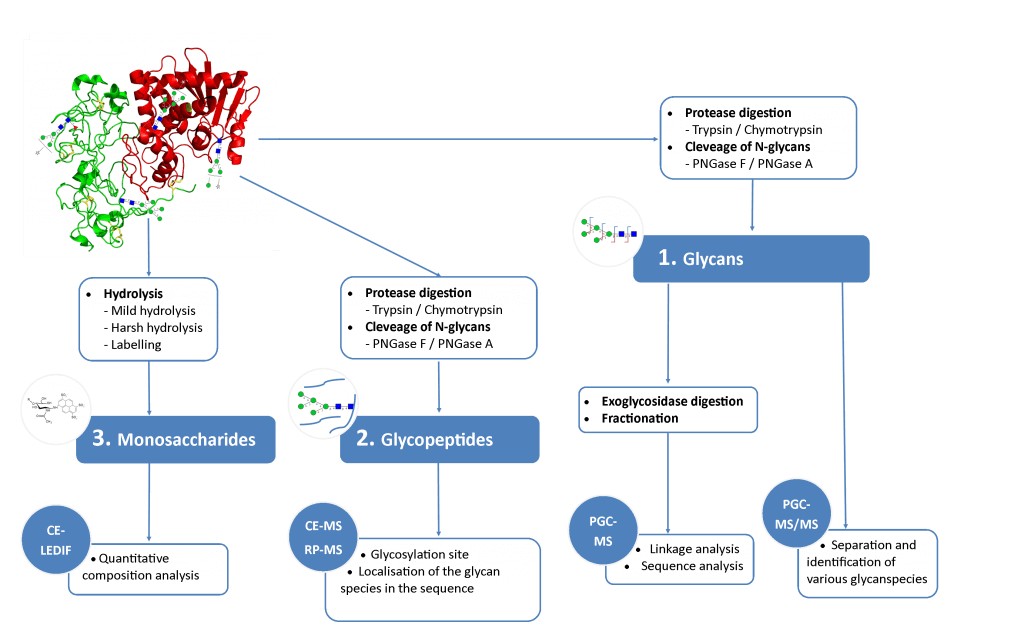
2 Methods
To identify the glycan species contained in the glycoproteins, separation of the glycan species was carried out using porous graphitized carbon chromotography (PGCC) and detection was implemented using an electrospray ionization quadrupole time-of-flight mass spectrometer (ESI-Q-TOF-MS). A capillary electrophoresis device was coupled to the mass spectrometer (CE-MS) for the analysis of proteolytically digested glycopeptides. In addition, a CE method was established for the analysis of the monosaccharide composition of glycans. The monosaccharides were separated by capillary electrophoresis and detected by means of LED-induced fluorescence (CE-LEDIF). Appropriate procedures for sample preparation were developed for all methods used. The glycan species were evaluated with the software GlycoWorkBench (GWB).
3 Results
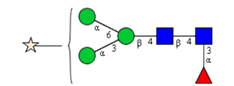
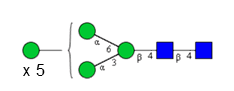
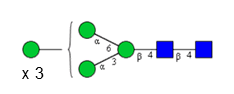
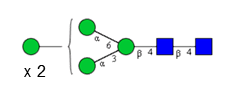
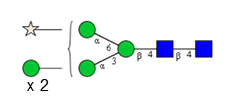
Sixteen different glycan species of the murine mAb and nine different glycan species of ricin were identified using PGCC-MS analysis (Tab. 1). Linkages of the monosaccharide building blocks of the glycans were determined for the murine IgG with the exoglycosidases sialinidase A and β(1-4,6)-galactosidase, and for ricin in part with α(1-2)-mannosidase and α(1-6)-mannosidase.
Table 1: Overview of the nine glycan species of ricin digested with chymotrypsin and PNGase A, identified using PGCC-MS; evaluation with GWB4 Conclusion
PGCC- and CE-based methods coupled with mass spectrometric or fluorescence detection enabled identification of the complex-type glycans of the murine IgG and the high-mannose type glycans of the plant toxin ricin.
[ratings]
Most importantly Prot pi | Protein Tool was used to calculate masses of proteins, glycosylated and deglycosylated, proteolyticaly digested peptides and glycopeptides. This tool has been enormously helpful and timesaving.