Overview
Nitration is a post-translational modification of mostly tyrosine residues that is caused by one-electron oxidation. First, a tyrosine radical is formed by one-electron oxidation followed by a reaction with nitrogen dioxide resulting in 3-nitrotyrosine.
| pKa | NC | Loss | Gain | Deltamass | H | AA | UV-Spec | Pattern |
| Acidic 7.25 | No | H | NO2 | Av: 44.9976 M: 44.9851 | – | Y | Yes* | – |
*For the calculation of the molar absorption coefficients the UV/Vis spectrum of 3-nitrotyrosine was taken.
In-depth mechanism
In contrast to many protein modifications, nitration is a naturally occuring chemical and not anenzymatic process. Through nitration, a nitro group is covalently attached to the carbon in 3-position of the aromatic ring of tyrosine residues forming 3-nitrotyrosine1–6. Recent studies suggest that the mechanism of tyrosine nitration is mediated by free radical reactions. In a first step, a tyrosine radical is formed by one-electron oxidation. Common oxidants for this reactionare hydroxyl, nitrogen dioxide, carbonate, peroxyl and alkoxyl radicals as well as oxo-metal compounds and compounds I and II of hemoperoxidases2,3. The tyrosine radical can then react with a nitrogen dioxide radical forming 3-nitrotyrosine1–5. Although this reaction is favored, the tyrosine radical can undergo different reactions with other radicals such as hydroxyl, superoxideor nitric oxide radicals forming 3,4-dihydroxyphenylalanine respectively tyrosine hydroperoxide or 3-nitrosotyrosine. In addition, the tyrosine radical can react with another tyrosine radical forming 3,3’-dityrosine. It is important to note that these modifications can be reversed by reductans such as ascorbate or glutathione or by intramolecular electron transfer reactions with other amino acids such as cysteine2,3. It is assumed that the denitration is mediated by enzymes3. The abbreviated version of tyrosine nitration is shown in figure 1a. A more detailed mechanism together with an overview of reactions with other radicals has been compiled by Radi and is shown in the figure 1b3.
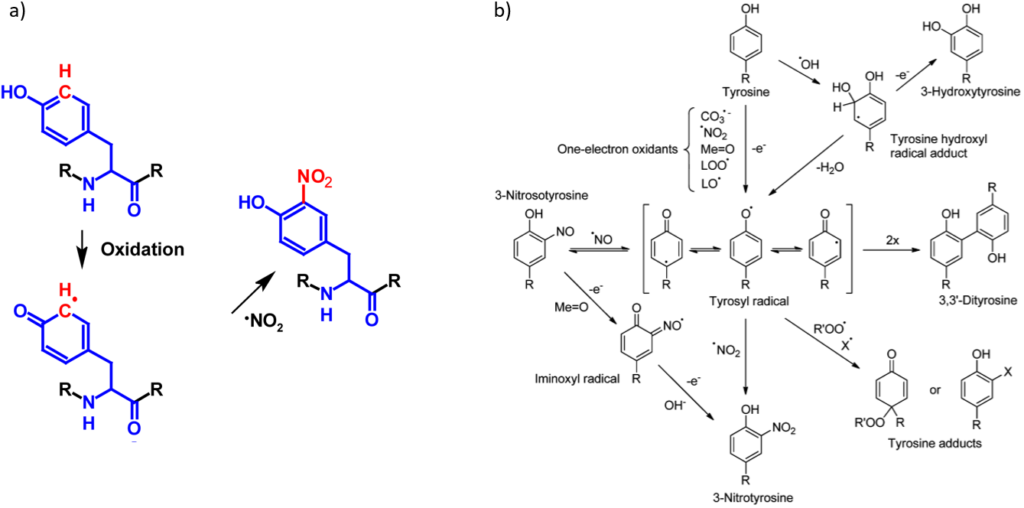
Tyrosine nitration increases the molecular mass of the modified protein by 45 Da and strongly affects the pKa value of the phenol. Through the neighbouring group participation of the nitro group the pKa is lowered from its original value of 10.0 – 10.5 to 7.0 – 7.51–6. This means that
approximately 50 % of the 3-nitrotyrosines are deprotonated while tyrosines are almost completely protonated at physiological pH. In addition, the nitro group changes the absorption behaviour of the protein in the UV range. The influence of the nitro group on the absorbance was determined by the difference of the UV spectra of tyrosine and 3-nitrotyrosine. The absorbance at 275 nm of 3-nitrotyrosine is approximately four times higher than of a non modified tyrosine residue. Moreover, similar to S-nitrosylation, 3-nitrotyrosine also absorbs light in the range between 320 – 450 nm7. Furthermore, the nitro group is a bulky and hydrophobic substituent which may cause local steric restrictions. This can trigger conformational changes and interfere with tyrosine phosphorylation1,3. Tyrosine nitration is a highly selective process. Generally only 1 – 5 out of 10’000 tyrosine residues are nitrated. However, there are some proteins where tyrosine nitration is more common2.
References
- 1.Abello N, Kerstjens HAM, Postma DS, Bischoff R. Protein Tyrosine Nitration: Selectivity, Physicochemical and Biological Consequences, Denitration, and Proteomics Methods for the Identification of Tyrosine-Nitrated Proteins. Journal of Proteome Research. 2009;8:3222–3238. doi:10.1021/pr900039c
- 2.Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox biology. 2018;14:618–625. doi:10.1016/j.redox.2017.09.009
- 3.Radi R. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Accounts of chemical research. 2013;46:550–559. doi:10.1021/ar300234c
- 4.Corpas FJ, Chaki M, Leterrier M, Barroso JB. Protein tyrosine nitration. Plant Signaling & Behavior. 2009;4:920–923. doi:10.4161/psb.4.10.9466
- 5.Turko IV, Murad F. Protein Nitration in Cardiovascular Diseases. Pharmacological Reviews. 2002;54:619 LP – 634. doi:10.1124/pr.54.4.619
- 6.Zhan X, Wang X, Desiderio DM. Mass spectrometry analysis of nitrotyrosine-containing proteins. Mass spectrometry reviews. 2015;34:423–448. doi:10.1002/mas.21413
- 7.Crow JP, Beckman JS. Quantitation of Protein Tyrosine, 3-Nitrotyrosine, and 3-Aminotyrosine Utilizing HPLC and Intrinsic Ultrviolet Absorbance. Methods. 1995;7:116–120. doi:https://doi.org/10.1006/meth.1995.1017